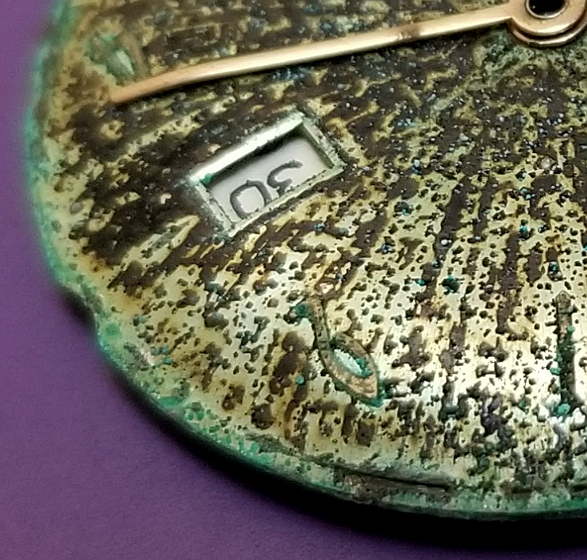"Trashed" TIMEX dial, with a beautiful pattern.
1965 TIMEX Marlin with a stunning radial pattern of corrosion.
Hi, this is Alan. thanks for looking. Contact info, below.
Well, I've seen many TIMEX dials, along with a great many of which were "degraded" in some way or another. But *this* dial is probably the most spectacularly degraded I have ever seen, creating an unintended (but not random) pattern of corrosion arranged in a radial pattern around the dial.
/end
Here is a sector of the dial. You can see that the predominantly brownish colored corrosion is arranged in a radial pattern, with straight lines of corrosion extending from the center of the dial to the periphery. You can also see tiny dots of green corrosion scattered throughout. (The green at the edges may be from corrosion of the case, rather than the dial, with the corrosion secondarily deposited on the dial edges, but originating from the case where it meets the dial.)
Many watch dials are made with tiny, straight grooves on the surface of the dial, to reflect the light in a kind of "sunburst" or "sun ray" pattern. As a desired aesthetic effect. I believe these tiny grooves served as "channels" along where the corrosion was deposited, creating this striking pattern.
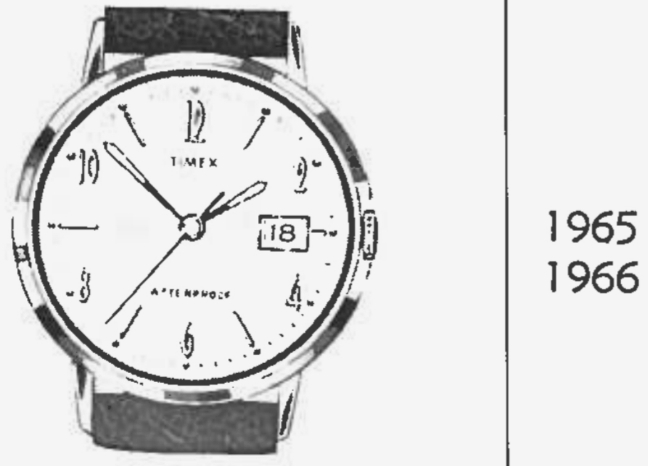
Although the hands of my watch are different from the watch pictured in this TIMEX repair guide, the dial is identical, with same style of lettering for the hours, going by even numbers, with hash marks for the odd numbers. Along with a date window. The second hand of mine has fallen off, and the hands of this pictured watch are flared, not straight, but otherwise identical. So, I'm putting my watch at ~ 1965. So, this is a "date version" of the TIMEX Marlin model, 1965.
So, what the heck has happened to this dial, since 1965, to create this appearance? I can generically describe what I'm seeing as "corrosion," the conversion of a refined metal to a more stable form, such as an oxide, hydroxide or sulfide. A chemical reaction(s) causes gradual destruction of the metal, and we see it as corrosion. The corrosion in iron is well known as rust, which is also called iron oxide, but most metals appear prone to corrosion of some form.
When you think of the destruction of something as changing to a more stable form, that's kind of awesome, isn't it? But we also know that when a radioactive element changes to a more stable form, it is described as having decayed. So, there is this concept of stability, through gradual self-destruction. How metal is that.
So, can we determine the composition of the metals in/on this dial? I'm not sure I can, but I'm trying. If you have any clues let me know. Many dials are brass (copper + zinc.) Copper corrosion is green. Zinc is less prone to corrosion, but when it does the corrosion is said to be white. Nothing in brass should be expected to produce a dark brown, rust-like corrosion.
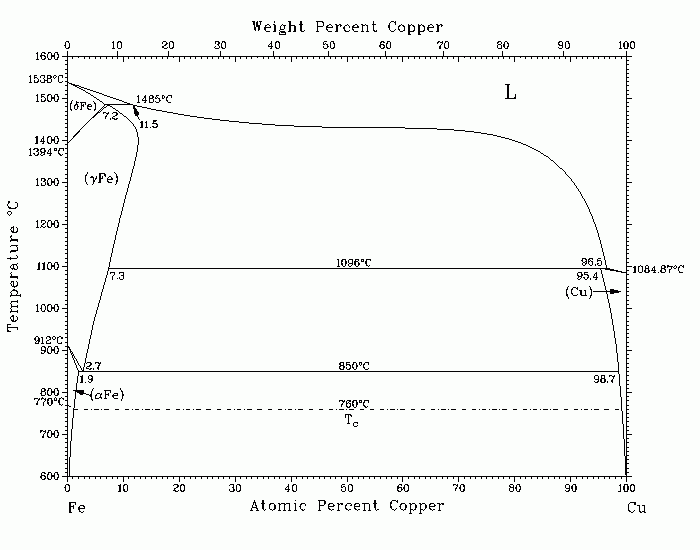
Maybe the dial is made from copper and iron? I looked up copper-iron alloys. It's possible, but almost unknown, except outside of experiments. Certainly not a well-known commercial product. See below, the phase diagram of copper. It is said to show how the melting points of copper and iron make it difficult to make an alloy, only a very narrow temperature window.
But what is more likely is that a brass dial has been plated with something else. And maybe that something is corroding brown? But what? Timex would not have used gold or silver on a $10 watch. Maybe chrome plating? Chrome is said to oxidize, from oxygen in the air to form chromium (III) oxide. But I get a sense that this isn't thick, brown like iron, and is described as forming a thin layer, that is actually self-protecting from further oxidation. Indeed, chromium is added to iron to make stainless steel, because it prevents, or at least limits the corrosion of iron.
So, I can't really explain the thick, long, dark brown radial lines of corrosion on this dial. I can't account for any reason this dial would be rich in iron. I also don't know any other metal that make such a characteristic color of corrosion. Any thoughts, let me know.