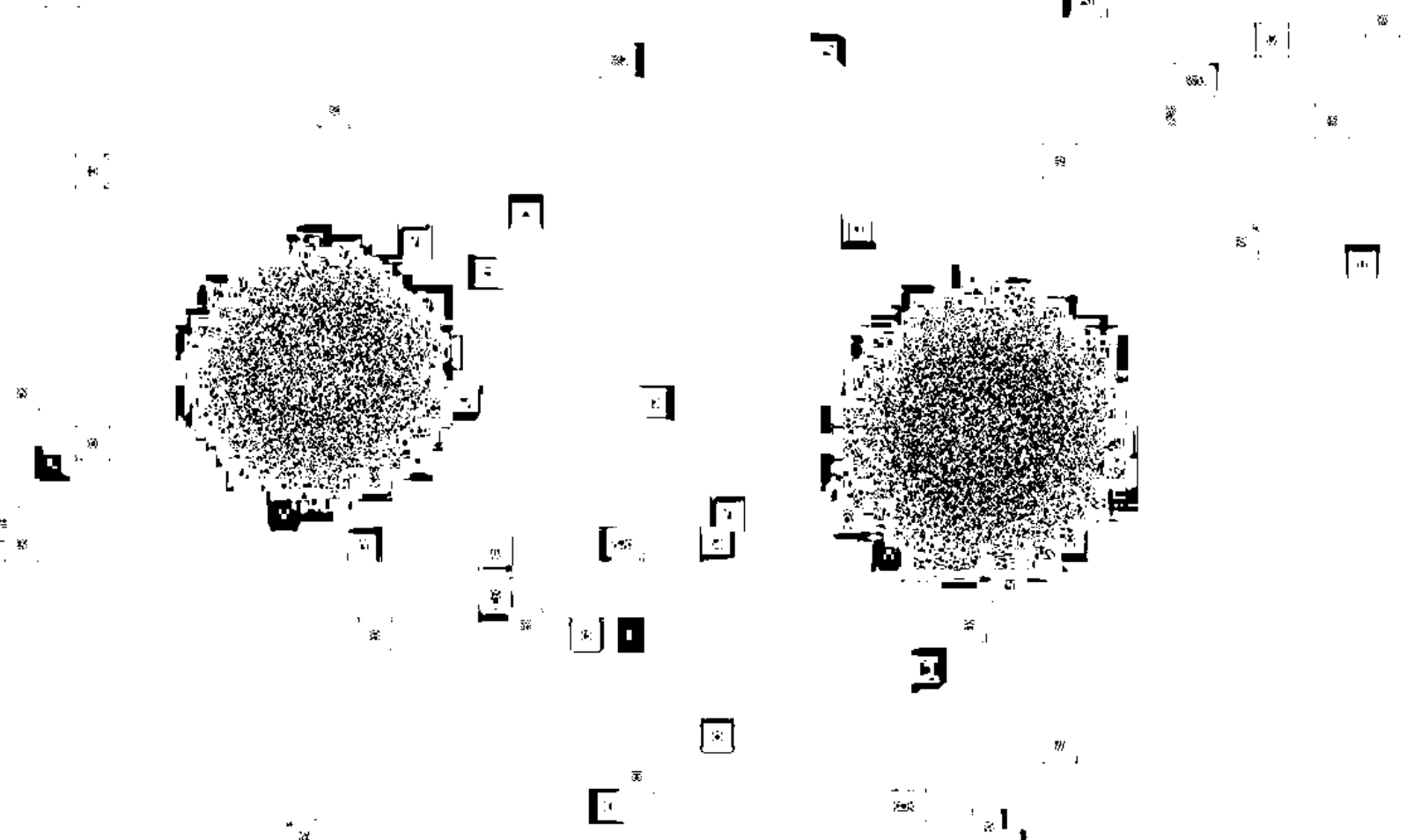
Radium watch dial image patterns by x-ray exposure
Autoradiograph of a 1945 Omega wristwatch by Alan (email).
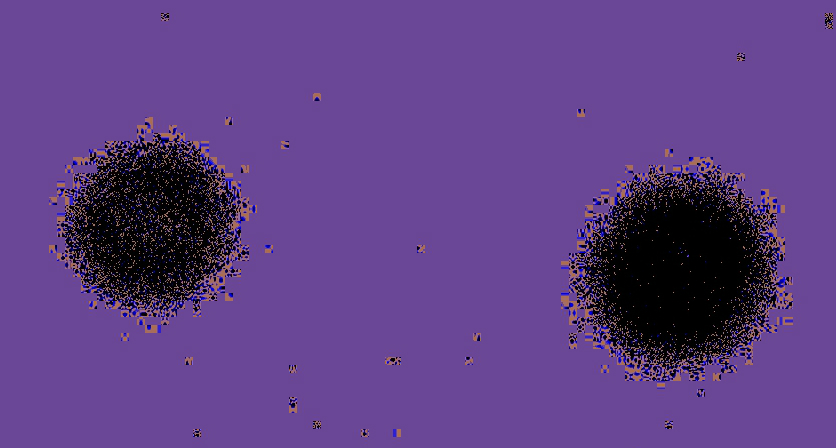
The above radiograph was obtained using the below radium dial Omega US Army watch (30SCT2-16,) exposed for 10, 20, and 30 minutes (L to R). [A bit far below about these old watches, "radiation," safety, etc.]
I knew the dial and hands were radioactive, after testing with a survey meter (Here's a vid of a different watch getting a survey.) The watch was placed face down on a standard computed radiography (CR) cassette for 10, 20, and 30 minutes in different but nearby locations on the cassette. The cassette was then run through the CR reader, and the resulting digital image was sent to the PACS workstation.
Once on the PACS, the data can be manipulated in a number of ways, resulting in wide differences on how the patterns appear. There is however one drawback to the CR, vs old fashion x-ray film, at least for this purpose, and I'll cover that below.
The image at the top of this page had to be windowed and leveled to extremes, to bring out the 10 minute exposure, which appeared quite of faint, unless you applied these extremes. This also amplified the effects of the background cosmic radiation strikes on the plate, all those "snowflake" splotches here and there. They are present, but less accentuated on the other images.
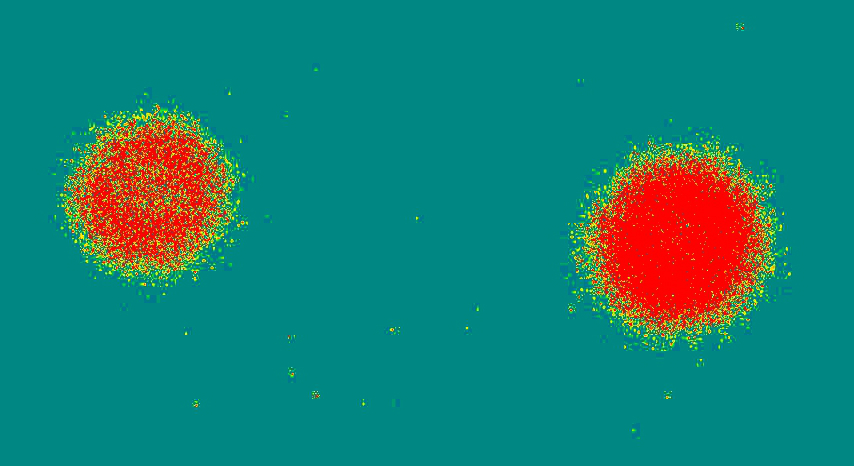
Above, showing just the the 20 and 30 minute exposures, processed through a color lookup table application built into the image processing tools of the PACS (not Photoshop, or other raster editing program). It is clear that with greater exposure time, the size of the exposure pattern enlarges. I was hoping, though, to be able to resolve the hands, and the individual 1-12 hour markers, all of which contain the radium paint. See the larger image below. This was obtained some years back, when traditional film-screen radiography was still being used. I went into a darkroom, removed a sheet of unexposed film from the drawer, and laid a different watch directly face down on the sheet of film. You can see the hands and hour markers are clearly resolved.
The problem with the CR method is that you are exposing the phosphor plates of the CR from a distance. Not a great distance, but enough such that the inherent geographic distortion that increases with distance comes into play. The few millimeter thickness of the cassette alone is enough to cause this. With film, the distance was nearly zero (some distance, as the crystal was still there). You could try to remedy this, by taking the cassette directly into a darkroom, opening it, up and laying the watch directly onto the photosensetive phosphor, but 1) my hospital has for a long not had a darkroom, and 2) there is some risk of possibly damaging the phosphor, leading to artifacts when the cassette is later used for patient purposes.
Above back to my old Omega, another view using a different a drop down choice, from the menu of the color lookup table, this one preset for imaging PET scans, once again 20 and 30 minutes.
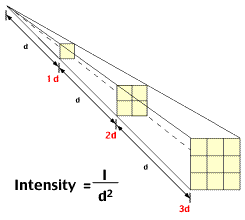
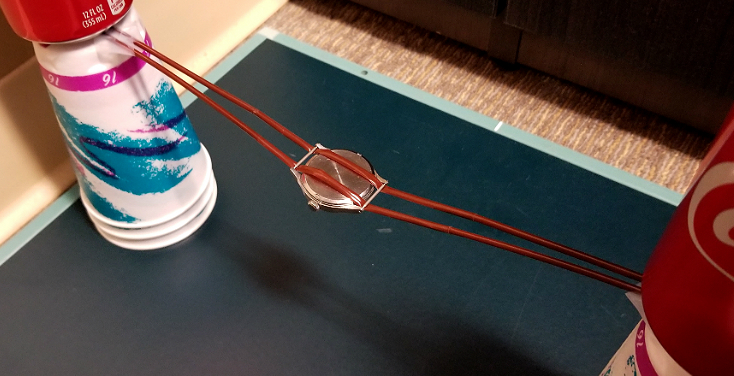
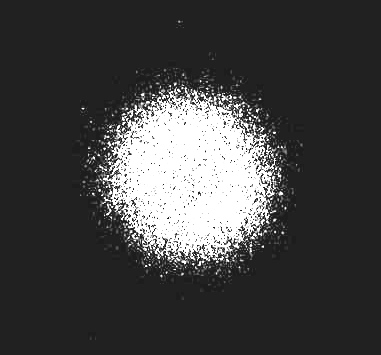
The below picture shows the set up from a slightly different experiment. Instead of laying the watch directly on the cassette, I suspended it 12.5 cm above the cassette (using six end-to-end coffee stirring straws, cup towers, and Coke cans for weight.) I wanted to see how the appearance would differ. I ran the experiment for five hours, instead of 10, 20, and 30 minutes, because of the expected diminishing effect of the radiations on the plate, with distance, according to the inverse square law.
Above is the result of this five hour experiment. Although I expected a far less contained and sharp image, I didn't expect this diffuse an image. There is a vague focus of greater exposure the center in a roughly round shape, but it is poorly defined. Both the inverse square law, as well as the effects of geographic distortion are responsible.
<<< Old radium dial watch laid directly onto x-ray film in a darkroom. From my recollection, the exposure time was about 15 minutes. The crystal was present, so there was some distance.
I can't remember what application brought out this appearance, but I think it was some kind of edge-enhancement filter. Again, 20 and 30 minute exposures are shown. Once more, the errant areas of exposure outside the region of the watch are probably strikes from background cosmic radiation.
What does any of this mean. Why do such an experiment. It was already clear the watch was radioactive from testing it with a survey meter, what does this add. Maybe not much, but I like visuals, and I'd done this before with traditional film many years ago, so I wanted to have another go at it. But also, these watches contain radium-226, with a half life of 1600 years.
Principal Modes of Decay (MeV):
Alpha 4.78 (94.5%), 4.61 (5.55%)
Gamma 0.186 (3.5%)
Much of the discussion I've read about radium concerns the potential accidental incorporation of the radium itself into the body, either from ingestion, inhalation or absorption. This certainly can occur, especially if you open up the watch, as over time the paint on the dial and hands can break down and create dust particles that can be breathed in, absorbed, etc. Watchmakers, for example, would be at greater risk of this than casual users of the watch who don't tinker with it or smash the crystal. (more below)
This isn't an autoradiograph, but a photograph of an old, radium dial watch. I had to shine a bright light onto the dial at very close range in a pitch black room. Then I switched off this bright light, and then very quickly took a photo before the glowing dial faded. Why did I have to do this, why isn't the dial glowing on its own, like it's supposed to.
Although the radium is still very active (half life 1600 years) the other component which is responsible for the glow, the photoscintillating material that glows green in in response to the radium decay, has gone "dead." Apparently this stuff chemically changes with time, such that it is no longer is strongly photoscintillating, and even though the dial is still very radioactive, it no longer glows on its own. But by blasting it with white light, some molecule or molecules absorb some of the light, converting the molecule into a very short-lived excited state. This then "reflects" back the absorbed energy as a kind of greenish light, as it goes back to ground state. If you're quick, you can get a picture before it fades completely. If I'm not mistaken, this is a form of fluorescence; even though the material no longer scintillates, it has some properties allowing it to fluoresce.
If you want to see the watch that gave rise to this image, click here. It's probably from 1930s.
Incorporation of radium into your body can be pretty serious. Radium shares some chemical properties with calcium, and when absorbed into your bloodstream, it gets deposited in your bones. Your bones! There, it probably stays either "forever," or unless/until that radium gets back back into your bloodstream, like if your bones undergo demineralization from osteoporosis or other disease state, then maybe you could "lose" the radium that was stuck in your bones. But otherwise, the radium will probably remain in your bones for your lifetime. Search "Radium Girls," to learn about some consequences of ingested radium. (More, below.)
Employees of the U.S. Radium Corp. paint numbers on the faces of wristwatches.
So, aside from ingestion/absorbtion/inhalation of radium, what else should be considered?
Well, the other consideration is the effects of being exposed to the radiation coming from the dial, just from the watch itself. As in, you haven't eaten or breathed in any of the radium, but the higher energy radiations from the dial, resulting from the radium decay, will easily pass through the crystal and then be able to bombard whatever may be in its path, with consideration again of the inverse square law that described radiation intensity as a function of distance from the source. So, could exposure to these watches be "dangerous?" I will not attempt to answer this question on this page. I do want to stimulate thought on the matter, though.
At the moment, as far as I am aware, there is no regulation relating to the use of these watches (in the US, where I live.) This includes mailing, wearing, or disposing of these watches.
If you're in San Francisco, and you buy a watch from New York, the seller can just ship it in a nonshielded bubble wrap envelope, without labelling the envelope, without telling anyone. You can wear this watch. Nothing about it alerts anyone around you that it is radioactive. You can fly from SF direct to Singapore, on a 17 hour flight, sitting next to a woman who is in her first trimerster of pregnancy, with the watch on your wrist a few centimeters from her gravid uterus. For 17 hours. She didn't consent to this, and of course, no one is even aware of it. (more below).
Or you could be a pediatric physician, say a pediatric ENT, and all day long you see dozens of children spending time with that Omega strapped to your wrist, very close to their head and neck. Those kids and their parents have no idea what is on your wrist.
Could you be endangering airline passenger's pregnancy, or endangering the kids in your ENT practice?
Radiation effects on tissues can be categorized into those that are dose dependent, and those that are dose independent. Dose-dependent effects require the radiation dose to exceed a certain threshold value before effect becomes manifest. Below the threshold value, the effect will not occur. Many of the effects of nuclear blasts and radiation fallout are in this category. Dose-independent effects have no minimum value below which you are "safe" from the effect. In theory, a single photon striking a DNA molecule in one of your cells if you are very unlucky, could alter the DNA such that mutagenesis (a gene mutation leading for abnormal function and/or development) or carcinogenesis (gene mutation leading to dysregulated cell growth = cancer) could occur.
So, let's assume for a moment that the watch is capable of "causing damage." In the case of your co-passenger on the plane, she could have a child with a gene mutation that resulted in a "birth defect," whether this is a visible morphological abnormality, an inborn error or metabolism or any one of hundreds of others. In the case of the patients in your ENT practice, "carcinogenesis" could occur, for example in the form of a brain tumor developing in one of your patients.
Discussing this appears "controversial," or at least surrounded by a kind of manufactured controversy especially to those in the business of buying/selling of vintage watches. They generally don't want to hear about it, as they would fear that any potential regulation or restriction would impact their business (though there are plenty of lovely vintage watches that do not have radioactive dials to indefinitely support such businesses.)
I have only a few of these watches. I don't ever wear them because I cannot prove that they are not unsafe. What I am certain of is that they emit radiation in the high energy ranges - "ionizing radiation" -of the type that has been demonstrated to cause DNA breakage. I don't think it's fair to wear such a watch and expose people who have no expectation that a stranger on a plane or in a doctor's office, or day care center, or anywhere is wearing a radioactive source on their risk, exposing their bodies to this radiation. I keep these watches in metal containers, stored far away from where I or anyone in my family generally venture. I am told that in the US your state's Department of Natural Resources handles disposals of rad waste.
This discussion is incomplete. I may add to this page, if time permits. Thanks for looking.
Alan
Contact: email
/end
Click here, or on below pic, to make it bigger. Here the dial and hands of an old Timex were removed completely and laid on the film. No crystal. Distance = zero. This is why the radiation pattern of the hands and hour dots are super sharp. Doing this was probably unwise, as I may have contaminated myself.